Non-Public Data
Non-public APCD data are for advanced data users with information needs that cannot be met with public data or custom reports. If you do not have experience working with medical claims and are looking for APCD data, please visit Custom Reports.
How to Apply
Please request an application to get started. You can
preview
the content of the application
prior to completing in order to determine what information will be needed for submission.
If you are uncertain of whether the data request process is appropriate for your use case or have any questions regarding the application process, please submit a Data Request Inquiry.
Who is eligible to request Non-Public Data?
Types of data requesters include researchers, government entities, payers, providers/health systems, industry, and employers. Data requesters must be affiliated with an organization.
What are the requirements for receiving access to Non-Public Data?
All Non-Public Data releases must align with Georgia APCD objectives. Within the data request application, the requester must justify how the data use will benefit the public. Data requesters must also have the methodological expertise and security infrastructure to analyze and protect the data. Data requests should be limited to the minimum data necessary to answer a research question. Additional justification is required for certain data elements to ensure antitrust compliance and to protect sensitive data.
How much does Non-Public Data cost?
Costs vary depending on the type and quantity of data being requested. For more details on data access and application fees, see Data Access Fees.
Non-Public Data Request Application Content
Requesters of Non-Public Data will need to submit an application, including the following:
| Requester information |
|
|---|---|
| Project plan |
|
| Data specification |
|
| Expected outputs |
|
| Data privacy |
|
Additional application elements may include:
- A copy of an Institutional Review Board (IRB) protocol for the research
- A Data Use Agreement (DUA)
- A Data Management Plan
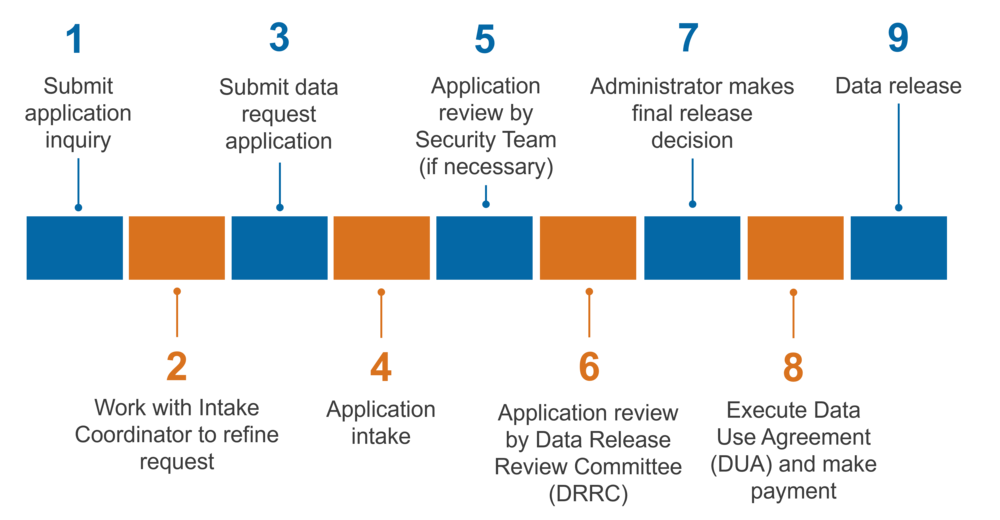
What is the process for requesting Non-Public Data?
What data elements are contained in Non-Public Data?
All data requests should be limited to the minimum necessary data. Most Non-Public Data releases will be fully de-identified. When necessary for the research topic, "limited identifiers" such as 5-digit zip codes or dates of service can be made available.
Some data may be classified as sensitive for a variety of reasons including antitrust regulations and sensitive conditions, medications, and procedures, or other criteria as defined by the Data Governance Planning Team.
Standard Modules
In order to simplify the selection of data elements from the APCD, we have developed a set of standard data modules, listed below. If you have data needs that are not listed here, please contact us.
| Data Module | Contents |
|---|---|
| Member Information |
|
| Member Coverage |
|
| Medical Claims |
|
| Medical Claims Costs |
|
| Pharmacy Claims |
|
| Pharmacy Claims Costs |
|
| Dental Claims |
|
| Dental Claims Costs |
|
| Provider Specialty |
|
| Provider Information |
|
| Provider Identifiers |
|
| Payer Type |
|
| Payer Identifiers |
|
*Notes
ZIP may be represented as ZIP-3 or ZIP-5 based on data needs. Dates may be represented as a full date or as a year and sequence within the year based on data needs. Payer, Provider, and combined Payer and Provider information may be limited based on requestor type.